Platelet aggregation in whole blood
Platelet aggregation using whole blood has the advantage of assessing platelet function in the presence of other blood cells.
Aggregation in whole blood can be assessed by measuring the decrease in single platelets by flow cytometry and by measuring the changes in impedance using specialised aggregometers.
Single platelet counting by flow cytometry measures platelet aggregation in whole blood as a decrease in the number of single platelets over time after stimulation with an agonist and is particularly sensitive to platelet micro-aggregate formation. The advantage of this approach is that the samples can be stabilised using a dedicated fixative, which allows the analysis to be carried out at a later stage.
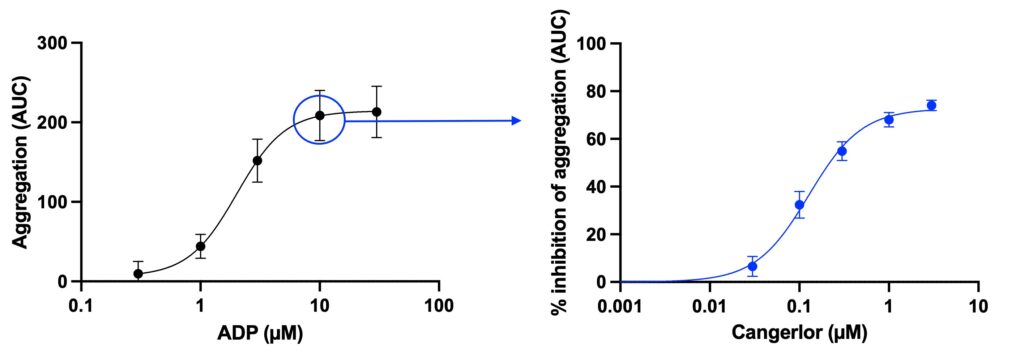
The samples can be fixed at several time-points providing data on dynamic aggregation response, similarly to LTA, with aggregation calculated and expressed as area under the curve (AUC). Alternatively, samples can be fixed at a single time-point offering a high throughput endpoint assay capable of generating numerous dose-response curves.
Use of whole blood provides the additional advantage of assessing platelet function in the presence of other blood cells. This can be particularly important for detecting potential indirect effects of agents on platelet function.
The principle is the same with both machines and is based on measuring the change in electrical impedance. Whole blood samples are placed into cuvettes and stirred with a magnetic stir bar. As platelets aggregate and accumulate on the electrodes in response to various platelet agonists, electrical resistance increases and this change in impedance is recorded over time.
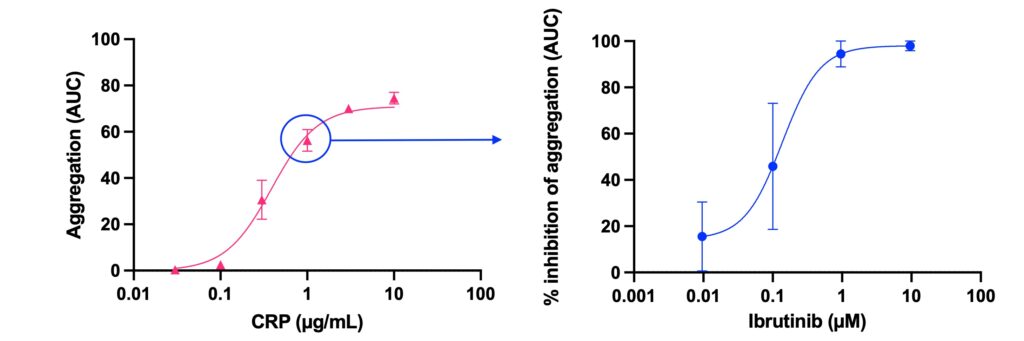
Aggregation response to a range of concentrations of collagen related peptide (CRP) measured in whole blood (WB) by impedance and presented as area under the aggregation curve (AUC) (left graph) and inhibition of CRP-induced aggregation (at 1.0 µg/mL) by ibrutinib, a tyrosine kinase inhibitor (dose response curve to ibrutinib, right graph).